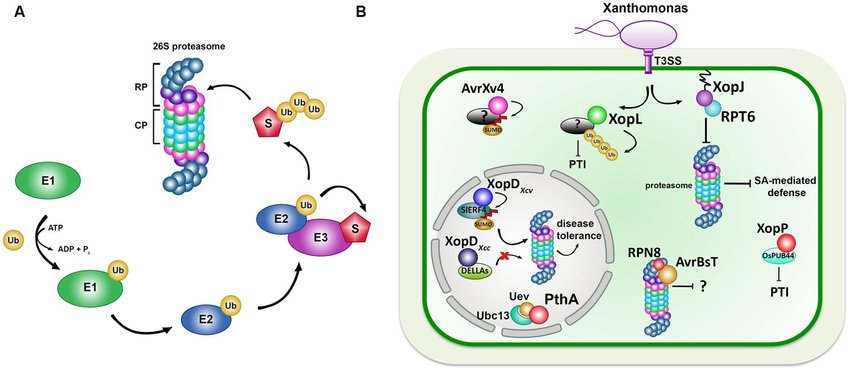
The ubiquitin-proteasome pathway is a crucial system responsible for degrading most intracellular proteins. It plays a significant role in various cellular processes, including programmed cell death (apoptosis) and protein degradation in neuronal function. This pathway involves the tagging of proteins with ubiquitin molecules, marking them for degradation by the proteasome, a large protein complex. The ubiquitin-proteasome system (UPS) is essential for maintaining cellular homeostasis by regulating protein levels and quality. Research has highlighted the UPS as a potential target for anticancer treatment, indicating its importance in disease therapeutics.
We delve deep into the intricate mechanisms and the pivotal roles played by Ubiquitin and the Proteasome in maintaining cellular homeostasis.
Ubiquitination: A Multistep Process
Ubiquitination proceeds through a cascade of enzymatic reactions involving three main components: E1 (Ubiquitin-activating enzymes), E2 (Ubiquitin-conjugating enzymes), and E3 (Ubiquitin ligases). These enzymes work collaboratively to attach Ubiquitin molecules to target proteins, determining their fate within the cell.
Diverse Functions of Ubiquitin
Once tagged with Ubiquitin, proteins can undergo various fates, including degradation by the proteasome, modulation of protein-protein interactions, regulation of protein localization, and even activation or inhibition of enzymatic activity. Such versatility allows Ubiquitin to influence a myriad of cellular processes, from cell cycle progression to DNA repair.

The Proteasome: Nature's Recycling Center
While Ubiquitin marks proteins for degradation, the Proteasome serves as the cellular recycling center, executing the demolition of tagged proteins with remarkable precision. Structurally, the Proteasome is a large, barrel-shaped protein complex comprising multiple subunits, each contributing to its function in protein degradation.
Proteasomal Degradation: An Intricate Choreography
Proteins targeted for degradation are recognized by the Proteasome, which then unfolds and translocates them into its central chamber, where they undergo enzymatic cleavage into short peptide fragments. These peptides are subsequently released and can be further degraded into amino acids by other cellular enzymes.
Regulated Proteolysis: Orchestrating Cellular Dynamics
The Proteasome's activity is tightly regulated, ensuring that only specific proteins are degraded at precise times and locations within the cell. This regulated proteolysis governs crucial cellular processes, including cell cycle progression, apoptosis, antigen presentation, and the removal of misfolded or damaged proteins.
Ubiquitin-Proteasome System: The Balancing Act of Cellular Homeostasis
The Ubiquitin-Proteasome System (UPS) operates as a finely tuned balancing act, orchestrating the delicate equilibrium between protein synthesis and degradation within the cell. Disruption of this balance can have profound consequences, leading to the accumulation of misfolded proteins, cellular dysfunction, and disease pathogenesis.
Implications in Disease: From Neurodegeneration to Cancer
Dysregulation of the UPS has been implicated in a wide array of human diseases, including neurodegenerative disorders (such as Alzheimer's and Parkinson's disease), cancer, inflammatory diseases, and cardiovascular disorders. Understanding the molecular mechanisms underlying UPS dysfunction holds promise for the development of novel therapeutic strategies.
Therapeutic Targeting of the UPS: A Frontier in Drug Development
The pivotal role of the UPS in cellular homeostasis has fueled intense interest in targeting this system for therapeutic intervention. Small molecules, peptides, and even biological agents designed to modulate UPS activity are being actively pursued as potential treatments for a range of diseases.
In the intricate tapestry of cellular life, Ubiquitin and the Proteasome emerge as central players, orchestrating the timely removal of unwanted proteins and maintaining cellular balance. Their partnership not only ensures the fidelity of essential cellular processes but also influences the development and progression of human disease. As our understanding of the Ubiquitin-Proteasome System continues to deepen, so too does the potential for novel therapeutic interventions aimed at restoring cellular homeostasis and combating disease.